A basic calcium compound (CaO), mainly used in industrial applications (cement, agriculture). In nutrition, it is less common due to reactivity, though its hydrated form (calcium hydroxide) aids pH regulation in certain formulations.
| Calcium Oxide | 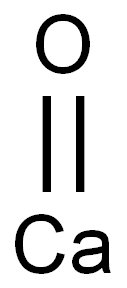 |
| CAS | 1305-78-8 |
| molecular weight | 56.08 |
| molecular formula | CaO |
| solubility | Solubility in water is 1.65 g/, with the risk of violent reactions. |
| color | White to yellow-slightly beige |
| flavor | Odorless |
| state | Powder |
| melting point | 2570 °C |
| boiling point | 2850 °C |
Calcium oxide (CaO), commonly known as quicklime or burnt lime, is a versatile inorganic compound with a long history of use across industries, from construction and agriculture to metallurgy and environmental science. Its alkaline properties, reactivity with water, and ability to form stable compounds make it indispensable in numerous applications. Here’s a breakdown of its key benefits:
1. Industrial and Construction: Foundation of Modern Infrastructure
Calcium oxide is a cornerstone of industrial processes and building materials, driving durability and efficiency:
Key Component in Cement and Mortar:
Reactswith silica and alumina to form the hydraulic binders in portland cement, the foundation of concrete used in buildings, roads, and bridges. Its inclusion ensures rapid setting, high compressive strength, and resistance to environmental wear.
In mortar and plaster, it combines with sand and water to create alkaline-resistant binders that adhere bricks and stones, enhancing the longevity of masonry structures.
Steel and Metal Production:
As a flux in metallurgy, it reacts with acidic impurities (e.g., silica, phosphorus) in iron ore, forming slag that is easily separated, thus purifying molten metal and improving the quality of steel, aluminum, and other alloys.
Drying and Dehumidification:
Highly hygroscopic, it absorbs moisture from air and industrial gases, serving as an effective desiccant in packaging (electronics, pharmaceuticals) and drying oils, fuels, and solvents.
2. Agriculture and Horticulture: Soil Health and Crop Protection
In farming, calcium oxide addresses soil imbalance and pest challenges, boosting agricultural productivity:
Soil pH Regulation:
Neutralizes acidic soils (low pH) by reacting with water to form calcium hydroxide (slaked lime), raising pH to optimal levels (6.0–7.5) for crop growth. This improves nutrient availability (e.g., phosphorus, magnesium) and reduces aluminum toxicity, which harms plant roots.
Calcium Fertilization:
Supplies calcium, a vital nutrient for cell wall structure, pollen germination, and stress tolerance in plants. It prevents disorders like blossom end rot in tomatoes and tip burn in lettuce, enhancing fruit and vegetable quality.
Pest and Disease Management:
Used in lime sulfur sprays to control fungal infections (e.g., powdery mildew, rust) and as a disinfectant in poultry farms and greenhouses, killing bacteria, fungi, and insect eggs.
Creates an alkaline environment in compost piles, accelerating decomposition and eliminating weed seeds or pathogens.
3. Environmental and Waste Management: Pollution Control
Calcium oxide’s alkalinity and reactivity make it a key player in environmental remediation:
Wastewater Treatment:
Neutralizes acidic industrial wastewater (e.g., from mining, steel plants, and power stations), adjusting pH to safe levels and precipitating heavy metals (e.g., lead, cadmium, zinc) as insoluble hydroxides for easy removal.
Enhances flocculation in water treatment plants, improving the efficiency of sedimentation and clarifying drinking water.
Flue Gas Desulfurization (FGD):
Absorbs sulfur dioxide (SO₂) emissions from coal-fired power plants and industrial boilers, converting it into calcium sulfite or sulfate—critical for reducing acid rain and meeting strict air quality regulations.
Landfill and Biogas Management:
Stabilizes organic waste in landfills by reducing odors and leachate toxicity, while boosting biogas production in anaerobic digesters by regulating pH for microbial activity.
4. Food and Pharmaceutical Industries: Safety and Functionality
Though less common than its hydrated form (calcium hydroxide), calcium oxide plays niche roles in food and healthcare:
Food Additive (E529):
Used in nixtamalization (processing corn for tortillas, masa) to enhance niacin bioavailability and improve texture, a traditional practice with modern safety standards.
As a pH regulator in food processing, it neutralizes excess acid in pickled goods and supports the production of certain cheeses and tofu.
Pharmaceutical Applications:
In veterinary medicine, it treats calcium deficiencies in livestock, while in human healthcare, it is used in some antacid formulations (indirectly, via calcium hydroxide) to neutralize stomach acid.
Acts as a drying agent in pharmaceutical packaging to protect moisture-sensitive drugs.
5. Chemical Manufacturing and Research: Versatile Reagent
Calcium oxide serves as a fundamental chemical reagent in laboratories and industrial synthesis:
Synthesis of Other Compounds:
Reactswith water to produce calcium hydroxide (slaked lime), a key ingredient in paints, adhesives, and paper manufacturing.
Used in the production of calcium carbide (for acetylene gas), calcium silicate (construction materials), and various calcium salts (e.g., calcium phosphate, calcium carbonate).
High-Temperature Reactions:
- 耐受 high temperatures, making it suitable for use in kilns, furnaces, and glass manufacturing, where it stabilizes the melt and reduces viscosity.
6. Safety, Sustainability, and Regulatory Compliance
Calcium oxide’s appeal is amplified by its natural abundance and industrial efficiency:
Abundant and Cost-Effective:
Mined from limestone (calcium carbonate) via thermal decomposition, it is widely available and economical, supporting large-scale industrial and agricultural applications.
Regulatory Approval:
In food and agriculture, its derivatives (e.g., calcium hydroxide) are recognized as safe by global bodies (FDA, EFSA), though pure calcium oxide is rarely used directly in food due to its strong alkalinity.
Environmentally Friendly Solutions:
Biodegradable and non-toxic in its reacted forms (e.g., calcium carbonate, hydroxide), it aligns with green chemistry principles, especially in pollution control and soil amendment.
Who Benefits from Calcium Oxide?
Construction and Infrastructure: Ensuring durable, long-lasting buildings and roads.
Farmers and Growers: Improving soil health, crop yield, and natural pest control.
Environmental Engineers: Providing cost-effective solutions for wastewater and air pollution.
Industrial Manufacturers: Enabling metal purification, cement production, and chemical synthesis.
Regulatory Compliance Teams: Meeting strict environmental and safety standards with proven reagents.
Calcium oxide’s versatility, reactivity, and foundational role in industry and agriculture make it a true workhorse of the modern world. From building skyscrapers to purifying industrial emissions, its ability to solve complex challenges with simplicity and efficiency cements its status as an essential compound across centuries of human innovation.
In sugar refining (e.g., from sugarcane or beets), calcium oxide is key to transforming raw juice into a clarified, processable solution:
pH Regulation for Impurity Precipitation:
Reactswith water to form calcium hydroxide (slaked lime, Ca(OH)₂), which neutralizes organic acids (e.g., malic, citric acid) in raw juice. This raises the pH to 7–8, causing negatively charged colloidal particles (proteins, pectin, gums) to coagulate and precipitate—critical for removing cloudiness and off-flavors.
In cane sugar refining, this step, known as “liming,” also precipitates metal ions (e.g., iron, magnesium), which can degrade sugar quality during evaporation or storage.
Enhanced Filtration Efficiency:
The formed calcium-based precipitates (e.g., calcium pectate, calcium phosphate) act as flocculants, trapping fine particles and facilitating their removal via filtration. This clarifies the juice, ensuring subsequent steps (evaporation, crystallization) work optimally without contamination.
Calcium oxide helps create a clean, concentrated sugar solution by targeting stubborn impurities that hinder crystallization:
Phosphate and Silicate Removal:
In beet sugar processing, calcium hydroxide reacts with naturally occurring phosphates and silicates to form insoluble calcium salts (e.g., calcium phosphate), which are filtered out. This prevents these compounds from interfering with sugar crystal formation or causing bitterness in the final product.
Color Precursor Destruction:
High pH conditions induced by calcium oxide break down color-forming molecules (e.g., melanoidins, caramelization byproducts) in the juice, reducing the need for aggressive decolorizing agents (e.g., activated carbon) later in the process. This preserves sugar purity and minimizes processing costs.
Sulfur Dioxide Synergy (in Sulfitation Processes):
In traditional sugar refining, calcium oxide is often used alongside sulfur dioxide (SO₂) to form calcium sulfite, which acts as a preservative and further inhibits color development. This “sulfitation” process protects the juice from oxidative degradation during evaporation, ensuring a lighter-colored sugar.
Calcium oxide indirectly enhances the final stages of sugar production by improving solution stability and crystal formation:
Controlled Evaporation Conditions:
By removing impurities early, clarified juice evaporates more uniformly, reducing the risk of scale formation (e.g., calcium carbonate deposits) in evaporation pans. This maintains heat transfer efficiency and extends equipment lifespan.
Promotion of Uniform Sugar Crystals:
Impurity-free syrup allows sugar molecules to align into large, uniform crystals during cooling, minimizing “molasses” (residual impure syrup) and maximizing sugar recovery. This is critical for producing high-purity products like white table sugar or pharmaceutical-grade glucose.
Calcium oxide’s alkaline properties make it a valuable tool in broader food purification and processing:
Starch and Protein Refining:
In corn starch or soy protein isolation, it adjusts pH to precipitate unwanted compounds, ensuring pure starch granules or protein isolates for use in food additives, adhesives, or textiles.
Water Treatment in Food Facilities:
Neutralizes acidic wastewater from canneries, breweries, or abattoirs, precipitating heavy metals and organic contaminants to meet environmental discharge standards. Its use here aligns with clean production practices, ensuring no harmful residues affect food safety.
Antimicrobial Surface Treatment:
In food storage and processing areas, calcium hydroxide (derived from calcium oxide) creates an alkaline environment that inhibits bacterial growth, particularly in facilities handling raw materials prone to microbial spoilage (e.g., fruits, vegetables).
Why Calcium Oxide is Indispensable in Sugar and Food Processing
By addressing three core challenges—impurity removal, pH stabilization, and process efficiency—calcium oxide ensures that sugar refining and food purification are both cost-effective and high-quality:
Technical Necessity: Enables critical clarification and decontamination steps that no other single reagent can fully replace.
Purity Assurance: Prevents color, flavor, and texture defects by eliminating contaminants early in the process.
Sustainability Alignment: Its use reduces reliance on synthetic chemicals for 脱色 and purification, supporting cleaner, more eco-friendly food production.
From the first step of juice clarification to the final stages of crystal formation, calcium oxide proves that even a simple compound can be the backbone of complex industrial processes—ensuring the sugars we consume are not just sweet, but pure, safe, and efficiently produced.